Recruitment for ConsCIOUS2 at ADHB has finished.
Thankfully, awareness during general anaesthesia with recall is very rare (1-2 in 1000). However, the ConsCIOUS1 study found that 4.6% of patients responded to the isolated forearm test (IFT), and the incidence in patients younger than 40 years was 11%. These rates suggest that connected consciousness occurs at a far higher rate than consciousness with recall.
The aim of the ConsCIOUS2 study is to provide further data for estimating the incidence of connected consciousness during general anaesthesia in healthy patients under 40, especially after intubation. We will also assess the cognitive capacity, check for associations between IFT response and post-operative delerium, and search for biomarkers using electroencephalography (EEG).
The Department of Anaesthesiology at the University of Auckland have received approval to gather data for the ConsCIOUS2 study at the Auckland District Health Board (ADHB: A+7854, HDEC: 17/NTB/194). The study is registered at ClinicalTrials.gov (Identifier: NCT03503357).
The study will be led at ADHB by Dr Matthew Moore, Dr Marta Seretny, and Dr Hanna van Waart, in partnership with Professor Alan Merry and Professor Simon Mitchell.

A primary aim of anaesthesia is to prevent awareness of surgery1, and ablation of the experience of surgery is the most secure way to prevent awareness with recall1. While the incidence of awareness with recall is very rare (0.1-0.2%2-4), a recent systematic review suggested that consciousness of intraoperative events may occur in approximately 37% of patients in experimental studies (as identified by the validated clinical procedure referred to as the isolated forearm test [IFT])1. However, Sanders et al recently found that incidence of consciousness with experience of the external world (connected consciousness) occurred in 4.6% of the general population undergoing anaesthesia5. The incidence in patients <40 years old was 11%. These rates, while lower than predicted by prior studies, suggest that somewhere between 1:20 and 1:10 patients may be aware under anaesthesia.
The IFT has been used for more than 40 years to identify intraoperative anaesthesia awareness in patients administered a neuromuscular block while under anaesthesia for surgery. It is a way of probing consciousness during anaesthesia and surgery in real time and, as such, is not dependent on recall. The test preserves patients’ ability to communicate by moving to a command even if they have been paralyzed to facilitate intubation. In the IFT, induction of anaesthesia is followed by inflation of a cuff on the dominant arm before neuromuscular blockade (paralysis) is induced. The cuff prevents paralysis of the hand allowing the patient to communicate to an observer through predefined hand movements, typically following a command like: “Mrs. Jones, if you can hear me, squeeze my hand”. This is then followed by further more complex commands such as: “Mrs. Jones, if you have pain, squeeze my hand two times” – to ensure the patient is comfortable.
To achieve our goals, we propose a multi-center prospective cohort study of a series of commands surveying responsiveness following laryngoscopy and intubation. Clinical care will not be controlled and will be left to the responsible clinician. We will also collect routinely recorded clinical data to frame the observations about IFT responsiveness, and additionally use non-invasive high-density electroencephalography (EEG) to provide information about the neural signature of IFT awareness. Finally, we will collect data on patient reported confusion and objectively measured confusion, to test whether IFT responsiveness has any association with these outcomes.
The aim of ConsCIOUS2 is to explore the cognitive state of the IFT responder, explore the long-term sequelae, and to use EEG to investigate possible neural signatures of IFT responsiveness.
Identify the incidence of IFT responsiveness following intubation in 18-40 year-old patients.
This is a prospective cohort study.
This is an international multi-site study. We will collect data from 50-70 patients at the ADHB, with a total of about 600 patients worldwide. The collection of data from 50 patients at Waikato Hospital is about to get underway.
It is a priority for us to run the study in a manner that minimises its impact on clinical practice. The largest impediment is likely to be the requirement for 20 or so minutes to apply the electrode net.
We will consult the elective lists for eligible patients coming in from ORDA, ignoring the patients being seen first. After consulting the anaesthetist, we will approach the patient with information on the study. When he/she had time to consider participation, we will return to seek consent. After obtaining consent, we will apply the cap in a consultation room in ORDA. The cap may also be applied in the back area of recovery.
Patient notes will be amended to reflect our approach and consent of the patient.
The intervention portion of this study is the assignment to undergo IFT testing.
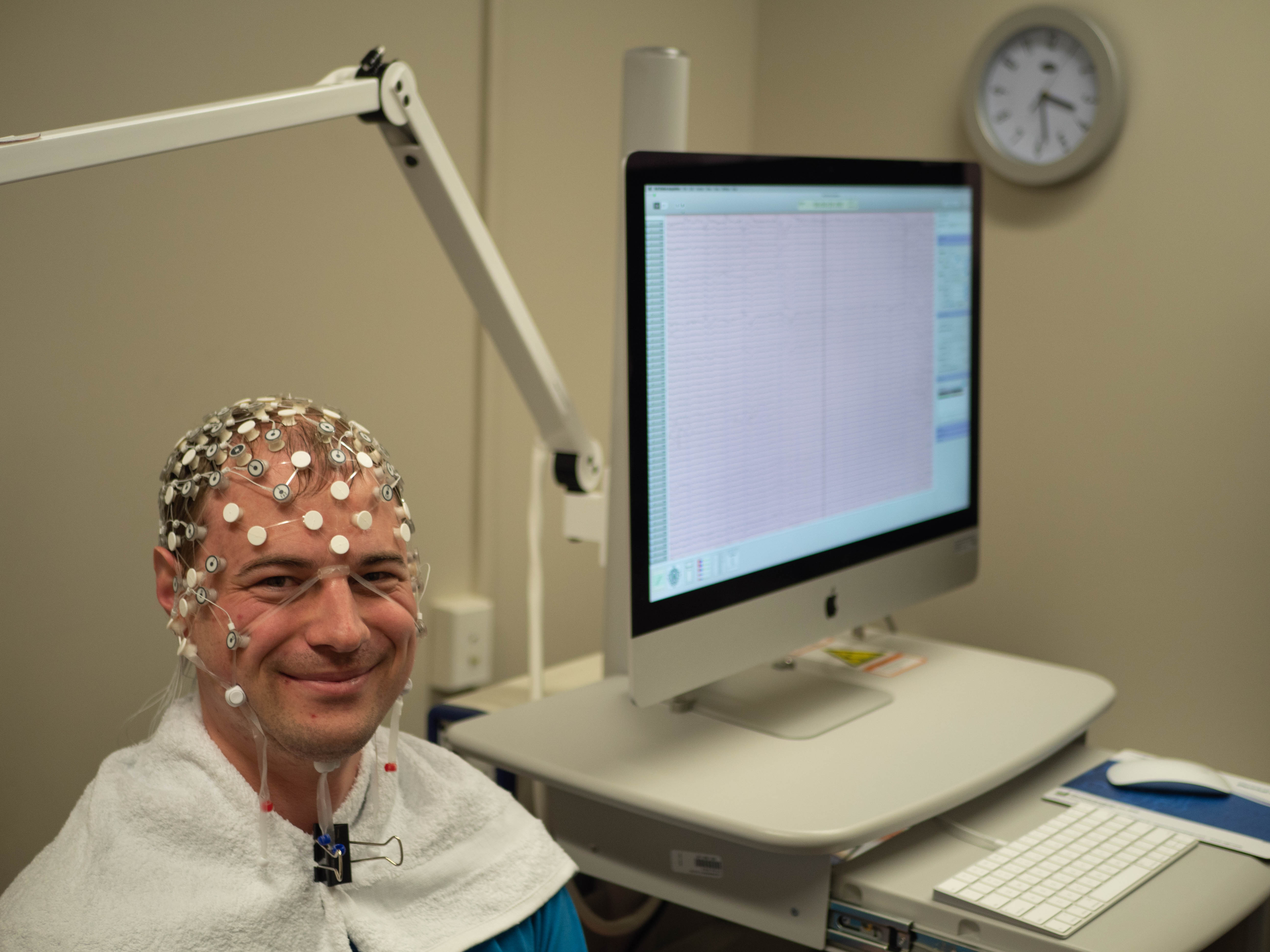

Site Investigators - Auckland |
|
|---|---|
| Matthew Moore | PhD, BE. Research Fellow, Department of Anaesthesiology, University of Auckland |
| Hanna van Waart | PhD. Research Fellow, Department of Anaesthesiology, University of Auckland |
| Alan Merry | FANZCA, FFPMANZCA, FRCA, FRSNZ. Specialist Anaesthetist Auckland City Hospital and Head of School of Medicine, University of Auckland |
| Simon Mitchell | PhD, FANZCA. Specialist Anaesthetist Auckland City Hospital and Professor, Head of Department of Anaesthesiology, University of Auckland |
